Therapeutic Innovation in Lung Cancer
“Advancing personalized treatment for people affected with lung cancer.”
DR. KARMELE VALENCIA LEOZ RESEARCHER. THERAPEUTIC INNOVATION IN LUNG CANCER RESEARCH GROUP
The Therapeutic Innovation in Lung Cancer group seeks to discover new potentially druggable targets and develop new therapeutic strategies to overcome both baseline and acquired resistance in these tumors. Our main objective is to advance in the development of new therapies or combinations that allow us to attack the tumor more effectively and offer real hope to patients who currently do not benefit from the available treatment options. We approach the problem from different angles to achieve a better and faster translation to the clinic.
Our focus is always on the sick person, being this the principle and foundation of our research, which seeks as a goal, to reach clinical impact.
We have many preclinical tools for the study of lung cancer that we have developed over more than 15 years of research in the field: cell lines, collections of patient samples or advanced murine models that recapitulate the human disease in its complexity, from early stages to more advanced stages with the presence of metastases.

Dra. Karmele Valencia
GROUP LEADER
| +34 948 194 700 | Ext. 81 2017 | |
| kvalencia@unav.es | |
| Research profile |
Oncology research integrated in the
Cancer Center Clinica Universidad de Navarra

Objectives of the Therapeutic innovation
in lung cancer Research Group
Identify new therapeutic targets with potential for clinical application.
To develop specific inhibitors for the newly identified targets.
To unravel the mechanisms of resistance to current therapies.
Explore novel therapeutic combinations to improve treatment effectiveness.
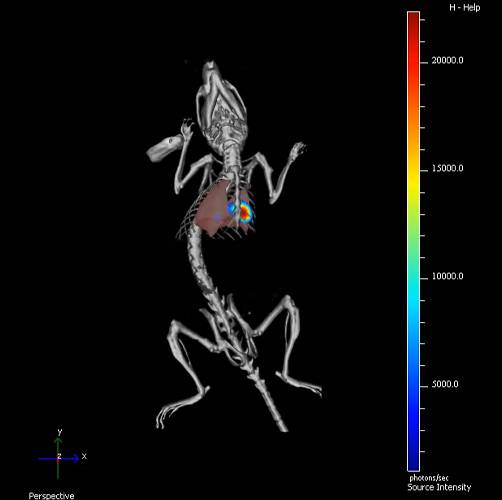
'Achilles' heel' of lung cancer found
We have discovered how DSTYK, a protein tyrosine kinase is altered in 6% of lung cancer patients. This alteration means a worse prognosis for these patients, as well as a lack of response to therapies based on checkpoint inhibitors (CPIs) and taxanes. The inhibition of DSTYK in these tumors leads to a resensitization of the tumor cells to the aforementioned treatments.

 Lines of research
Lines of research
PI: Karmele Valencia
Despite impressive progress in therapeutic strategies for non-small cell lung cancer (NSCLC), there are still many patients who cannot benefit from current therapies (chemo, radio, targeted therapy or immunotherapy).
We propose to identify new potentially druggable molecular alterations and evaluate mechanisms to break resistance to standard clinical treatments. Recently, we have published that DSTYK is altered in lung cancer and is associated with poor clinical prognosis. Furthermore, DSTYK controls cellular processes encompassing cytoprotective autophagy and mitochondrial welfare, and its inhibition sensitizes tumor cells to T cell-mediated death (Valencia et al. JEM, 2022).
Objectives:
In the present project we will characterize DSTYK as a novel biomarker and therapeutic target to sensitize lung cancer to standard treatments. In parallel, we will elucidate the intrinsic and extrinsic mechanisms related to DSTYK in lung cancer progression and, finally, we will identify selective DSTYK inhibitors with significant and specific antitumor activity at the cellular level to subsequently propose drugs that can be immediately taken to the preclinical phase. Our ultimate goal, if our hypothesis is confirmed, is to develop, in collaboration with our Medicinal Chemistry Department, a specific drug to inhibit DSTYK activity to treat lung cancer and potentially other cancer patients.
PI: Karmele Valencia, Álvaro Teijeira, Jon Zugazagoitia, Pilar Martín, José Martínez, Aránzazu González.
People with lung disease face a 12-fold increased risk of developing fatal heart disease compared to the general population, especially in the first year after diagnosis. Therefore, early detection, monitoring and cardioprotective treatments are critical. Two out of three lung cancer patients are diagnosed at advanced stages, in which immunotherapy, specifically with immune checkpoint inhibitors (ICIs), has become the standard treatment. However, ICIs carry the risk of immunological adverse effects, such as myocarditis, heart failure (HF), atherosclerosis and thrombosis, reinforcing the significant role of inflammation in cardiovascular damage associated with anti-tumor therapy.
Objectives:
The InCaRe consortium, composed of leading groups in lung cancer, cardiomyopathies and immunology, will lay the groundwork for the development of tools aimed at identifying patients with a high-risk immunological and cardiovascular profile prior to treatment. In addition, we will explore a comprehensive panel of molecular biomarkers capable of detecting early alterations induced by ICIs, linking them to disease progression and the possibility of predicting severe cardiovascular events.
The ultimate goal is to comprehensively assess cardiovascular damage in experimental models and lung cancer patients, identify molecular biomarkers of early cardiovascular damage, and explore potential anti-inflammatory therapies to mitigate the cardiotoxicity of immunotherapy. InCaRe will provide valuable insights into immune response and autoimmune activity in lung cancer patients receiving immunotherapy.
PI: Karmele Valencia Marta Montes, Oskar Marín, Patricia Altea-Manzano.
Lung cancer remains the leading cause of cancer-related mortality worldwide. Some people with lung cancer develop chemoresistance rapidly, leading to disease progression. Treatments, such as platinum-based chemotherapy and taxanes, stop cell proliferation in a small population of cells. This process, known as therapy-induced senescence (TIS), is considered a cellular escape strategy from the direct cytotoxic effects of treatment. We have found that prolonged treatment with platinum-based doublet chemotherapy increases senescent cells in non-small cell lung cancer (NSCLC) models both in vitro and in vivo, which correlates with increased resistance to therapy.
We hypothesize that there are specific mechanisms of therapy resistance associated with the presence of TIS cells, and that targeting senescence-induced mechanisms improves treatment response.
Objectives:
We will investigate how senescent cells influence surrounding cancer cells and non-malignant cells, increasing their aggressiveness, resistance to therapy and ultimately leading to tumor relapse.
Specifically, we will: (1) evaluate the impact of TIS on the tumor microenvironment, (2) characterize TIS in lung tumors and identify its escape mechanisms in the face of therapeutic pressure, and (3) evaluate new drug combinations to prevent the activation of senescence-related mechanisms.
With this project, we aim to elucidate the vulnerabilities of NSCLC tumors to define targets that modify TIS and propose new combination therapies to overcome resistance to chemotherapy.

LUNG CANCER
It is the leading cause of death from cancer worldwide.
Lung cancer accounts for approximately 12-13% of all new cancer diagnoses. The incidence and mortality associated with this type of cancer represent a major challenge for public health policies in advanced societies. It is estimated that by 2035, less developed regions will experience a 144% increase in new cancer cases, compared to 54% in more developed regions.
Typically, two out of three lung cancer patients are diagnosed at advanced stages, when curative options and survival rates are limited. In persons in stage IV, five-year survival is less than 15%. In early stages of the disease, the risk of mortality remains high, with relapse rates of 30-45% within five years of diagnosis. Despite major advances and efforts in personalized medicine, a significant proportion of the lung cancer population is unable to benefit from current therapies.
The lack of response or development of resistance to standardized treatments such as chemotherapy or immunotherapy and/or the absence of known therapeutic targets leads to a therapeutic failure that means the cancellation of therapy tools for the patient. This is one of the most relevant clinical problems in the management of the disease.
Meet the research team







Highlights....
Scientific activity of the Therapeutic innovation in lung cancer research group
Latest scientific publications
- Dual ENPP1/ATM depletion blunts DNA damage repair boosting radioimmune efficacy to abrogate triple-negative breast cancer Aug 13, 2025 | Magazine: Signal Transduction and Targeted Therapy
- DSTYK Inhibition Sensitizes Non-Small Cell Lung Cancer To Taxane-Based Chemotherapy Nov 11, 2024 | Magazine: Journal of Thoracic Oncology
- Protein Biomarkers in Lung Cancer Screening: Technical Considerations and Feasibility Assessment Jul 17, 2024 | Magazine: Archivos de Bronconeumología
- Low-dose ionizing γ-radiation elicits the extrusion of neutrophil extracellular traps Apr 17, 2024 | Magazine: Clinical Cancer Research