RNA and Senescence
“RNA represents an unexplored frontier in aging and senescence research. Its study may help us design strategies against senescence-associated diseases including cancer.”
DR. MARTA MONTES RESANO RESEARCHER. RNA AND SENESCENCE RESEARCH GROUP
Our group, integrated into the Cancer Center Clínica Universidad de Navarra, aims to generate knowledge and tools to identify and target senescent cells, improving cancer treatment and other age-related diseases.
We approach this from a less studied angle: the regulation of RNA, a dynamic, specific, and highly orchestrated process.
In close collaboration with clinicians and other renowned groups, we are developing innovative tools such as patient derived organoids.
Employing cutting-edge transcriptomic techniques, we aim to study alterations in RNA during senescence in cancer and aging.
Understanding the RNA-senescence-cancer axis will enable the discovery of novel therapeutic targets for senescent cell clearance. The rapid advancements in RNA-based therapies and the innovative technologies currently being developed make RNA a promising avenue for developing new biomarkers and therapies for different diseases.

Dra. Marta Montes
GROUP LEADER
| +34 948 194 700 | |
| mmontesr@unav.es | |
| Research profile |
Oncology research integrated in the
Cancer Center Clinica Universidad de Navarra

Objectives of the RNA and Senescenc
Research Group
Specific senescence markers
Identify new specific senescence markers that allow us to detect and target senescent cells.
Vulnerabilities
Discover vulnerabilities of senescent cells in cancer and aging.
Combining therapies
• Combine RNA-based therapy with current treatments to prevent senescence-associated diseases, including cancer.
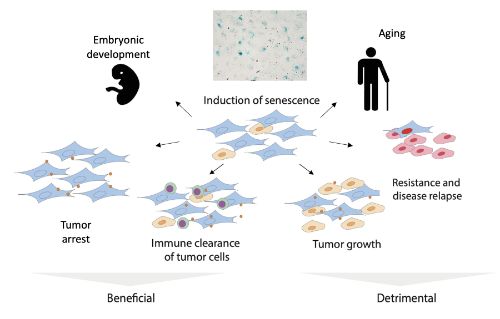
Senescent cells
Senescent cells are cells that have permanently stop dividing but remain metabolically active. Although initially considered a protective mechanism against cancer, the accumulation of these cells with age has been associated with various age-related diseases, such as cancer, cardiovascular disease, and neurodegenerative disorders. The fact that only a small percentage of cells are senescent in a tissue or tumor, along with the lack of specific markers for senescence, has hindered the development of methods to identify and study this population of cells.
 Lines of research
Lines of research
PI: Marta Montes
Long non-coding RNAs (lncRNAs) are long RNA molecules that do not code for proteins and are involved in cellular functions, both in physiological conditions and in pathologies. Due to their specific expression pattern, in our laboratory we are interested in studying these molecules as potential markers to detect senescent cells and as targets for their elimination.
Our objectives are:
- Identify lncRNAs that are specifically expressed in senescent cells
- Determine the functional role of lncRNAs in therapy-induced senescence
- Investigate the therapeutic potential of lncRNAs"
PI: Marta Montes
The deposition of chemical modifications on the RNA introduces functional diversity in the transcriptome beyond the DNA sequence. These post-transcriptional modifications on the RNA, termed “epitranscriptomics”, have emerged as novel regulators of gene expression. RNA modifications can influence RNA structure or RNA interactions with other molecules modulating RNA metabolism and function becoming an additional layer of regulation. We hypothesize that specific RNA modifications are important for the senescence response to chemotherapy treatment. Targeting the regulatory modification pathways in therapy-induced senescence will allow to improve cancer treatment.
The objectives of this line of research are:
- Identify specific RNA modifications and RNA modifiers that are deregulated in therapy-induced senescence in ovarian cancer.
- Characterize the molecular mechanism of the senescence-specific RNA modification in vitro.
- Investigation the therapeutic potential of targeting the senescence-specific RNA modification in vivo.
PI: Marta Montes
Recent research suggests a complex interaction between metabolic enzymes and RNA. Preliminary data from our laboratory has shown that DLST, an enzyme of the tricarboxylic acid (TCA) cycle, interacts with a specific lncRNA during oncogene-induced senescence. This interaction allows for the proper localization and function of DLST within the cell. Based on these previous studies in our laboratory, our hypothesis is that RNA, through its interaction with metabolic enzymes, contributes to cellular metabolic reprogramming in response to the induction of TIS in ovarian cancer.
In collaboration with national and international groups, our general objective is to identify the metabolic enzymes that interact with RNA in therapy-induced senescence and the metabolic consequences of such interaction.

Senescent cells are associated with various age-related diseases.
Senescent cells are cells that have permanently stop dividing but remain metabolically active. Although initially considered a protective mechanism against cancer, the accumulation of these cells with age has been associated with various age-related diseases, such as cancer, cardiovascular disease, and neurodegenerative disorders.
The fact that only a small percentage of cells are senescent in a tissue or tumor, along with the lack of specific markers for senescence, has hindered the development of methods to identify and study this population of cells.
Meet the research team



Scientific activity of the RNA and Senescence research group
Latest scientific publications
- A lncRNA-mediated metabolic rewiring of cell senescence [SP] May 21, 2025 | Magazine: Cell Reports
- The chromatin-associated lncREST ensures effective replication stress response by promoting the assembly of fork signaling factors Feb 1, 2024, | Magazine: Nature Communications
- A senescence-specific lncRNA controls metabolic rewiring of senescent cells Jan 26, 2024 | Magazine: bioRxiv
- Autophagy-mediated control of ribosome homeostasis in oncogene-induced senescence Nov 28, 2023 | Magazine: Cell Reports
